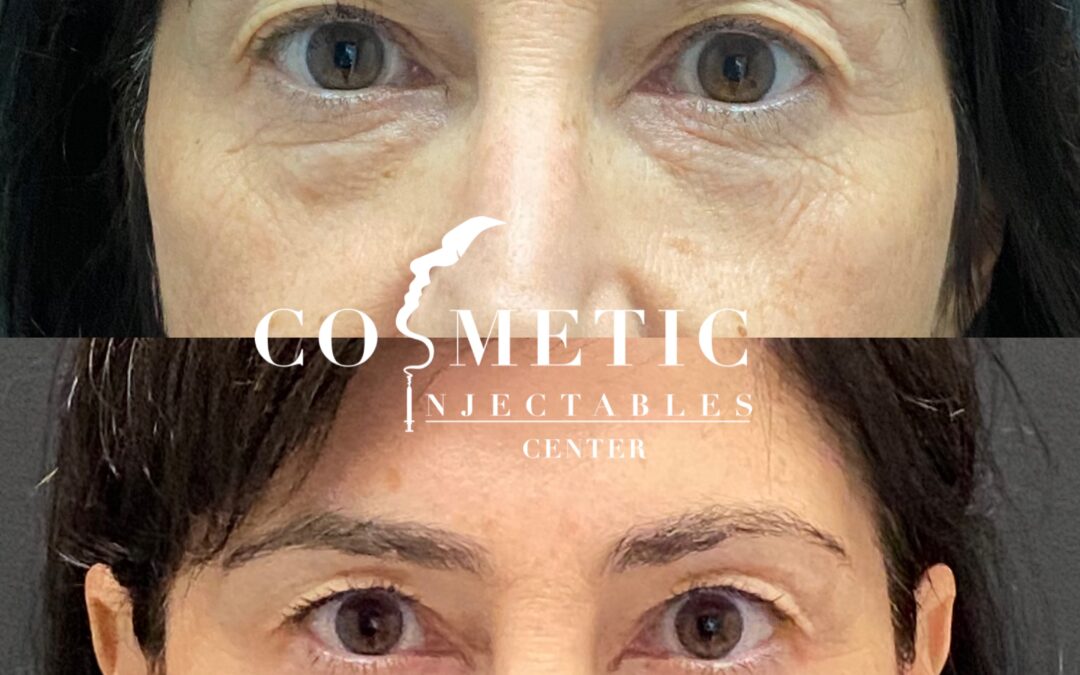
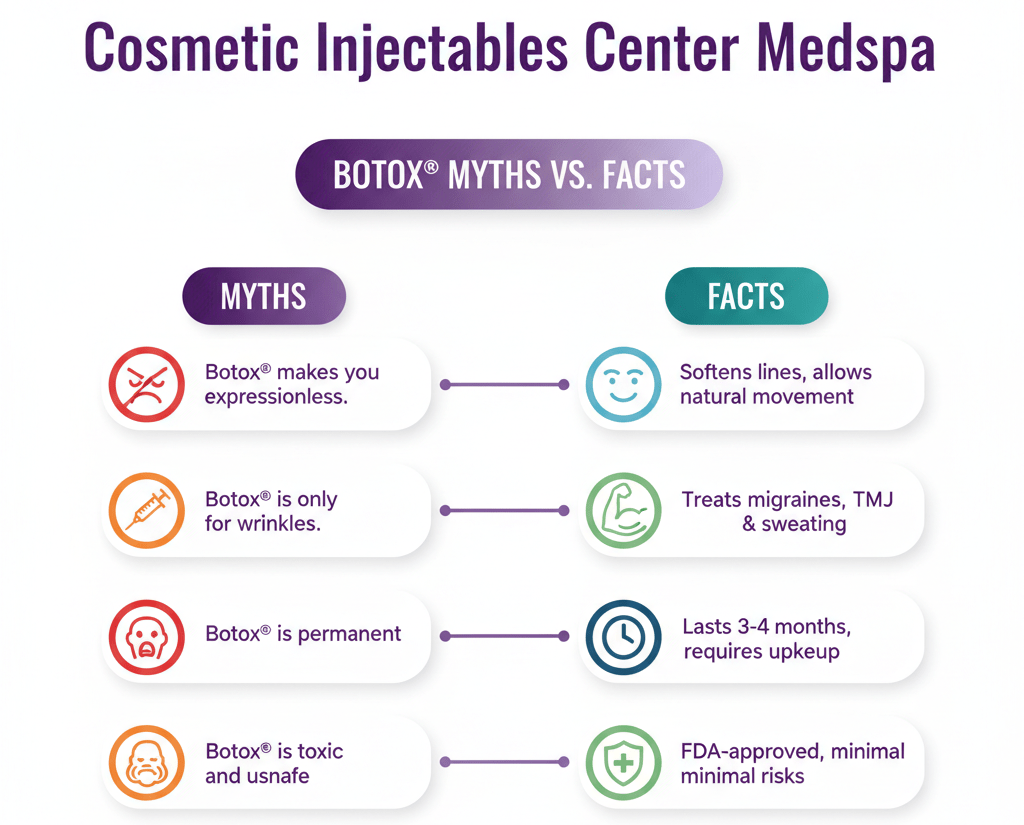
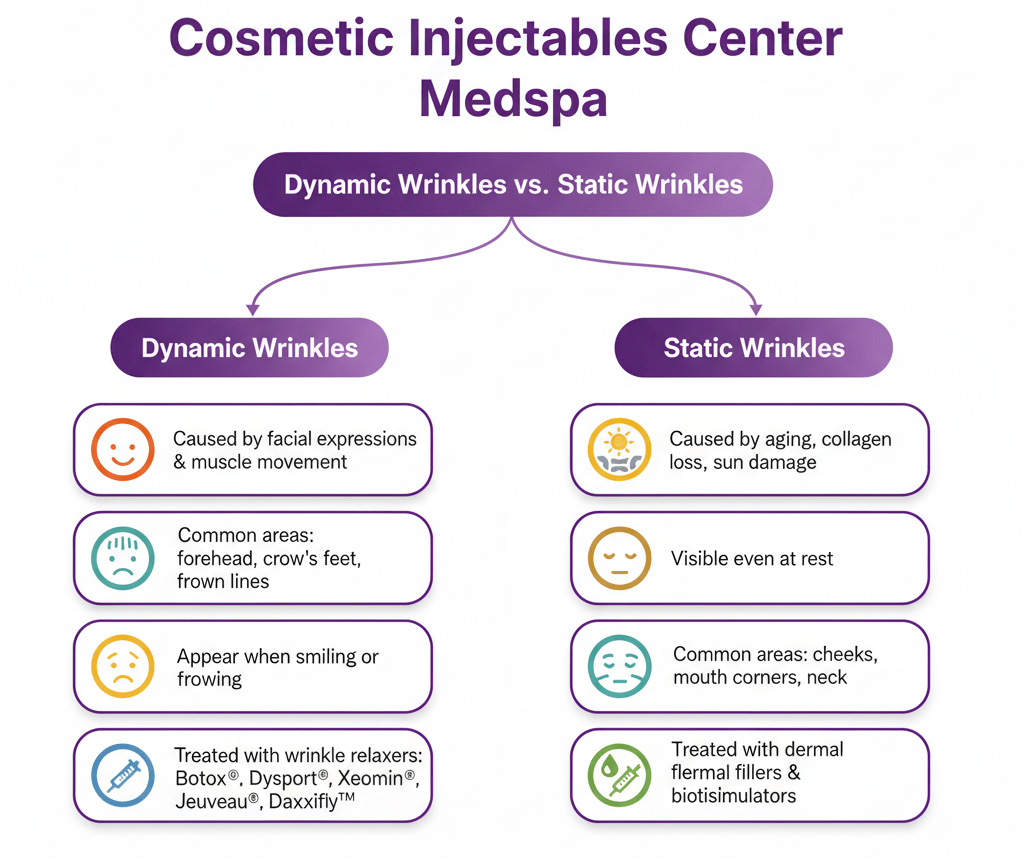
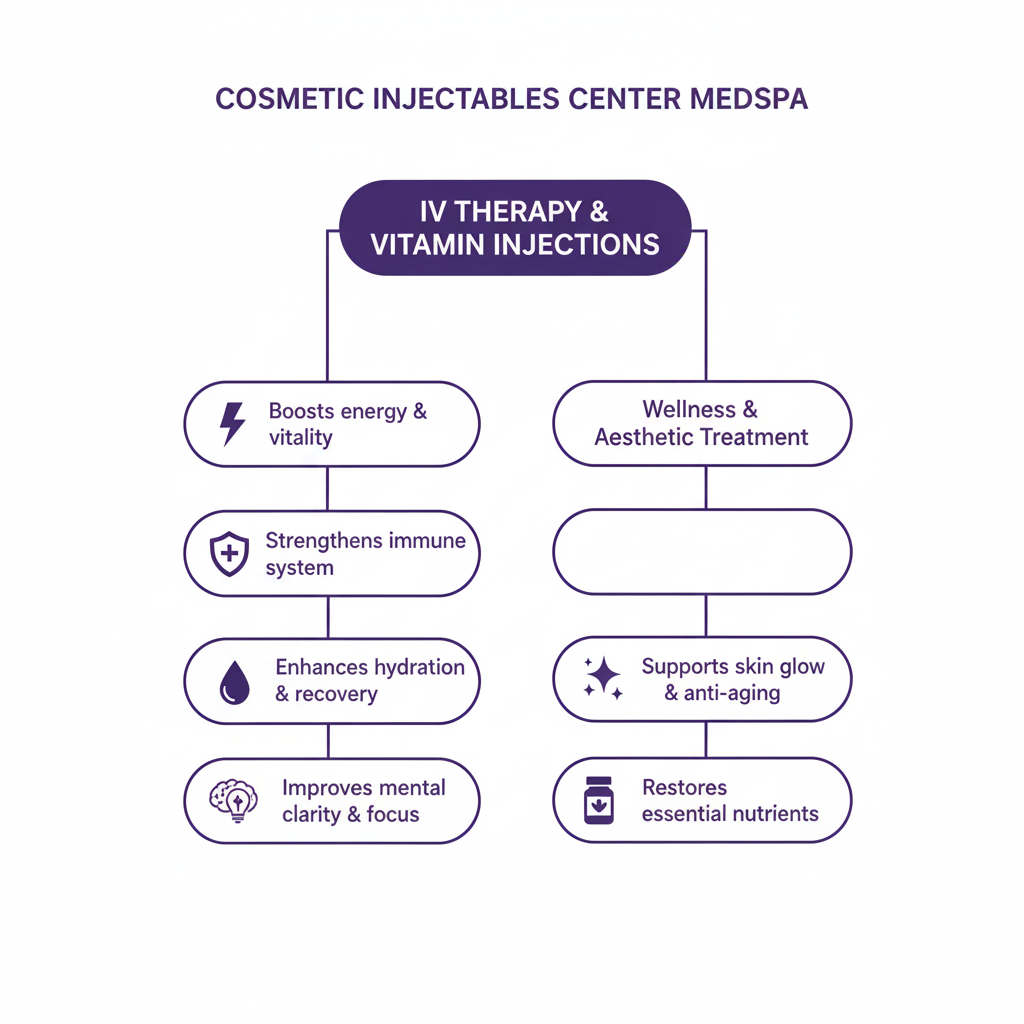
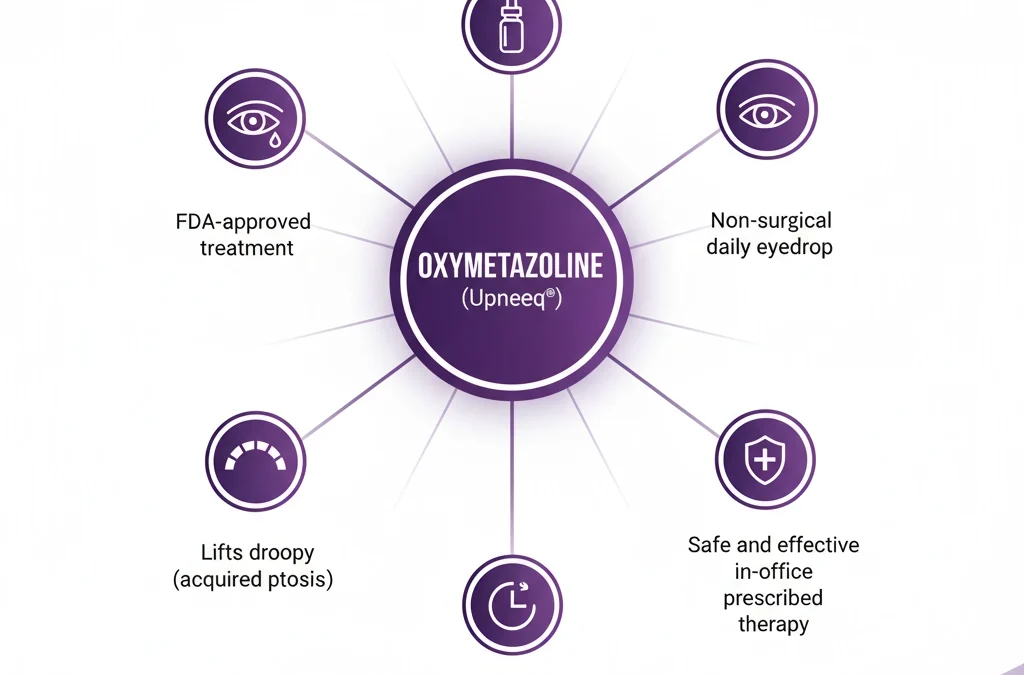
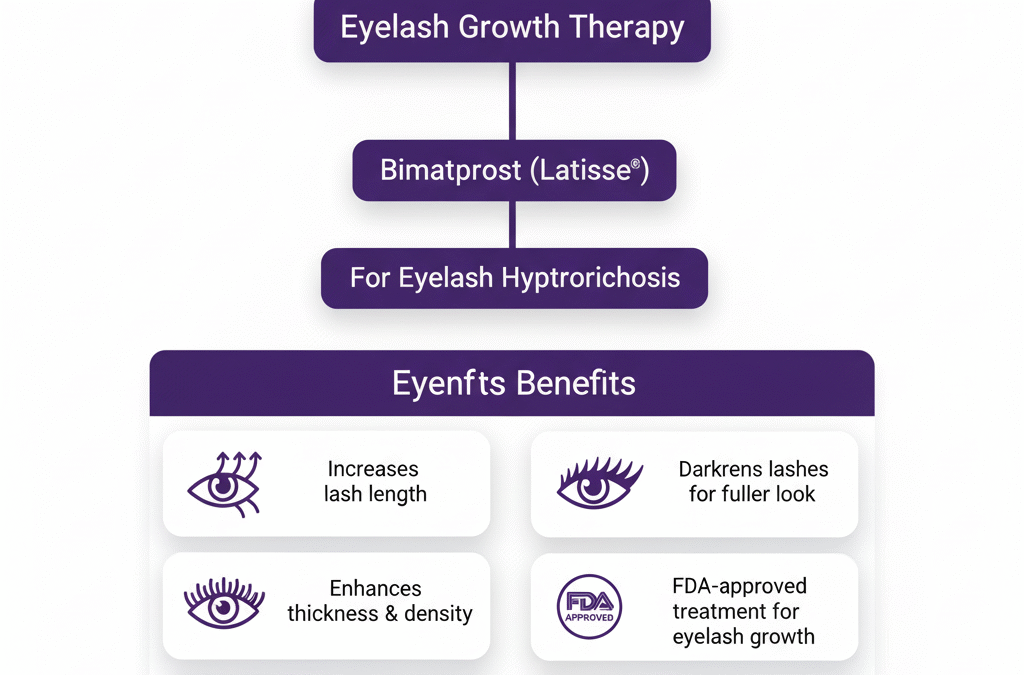
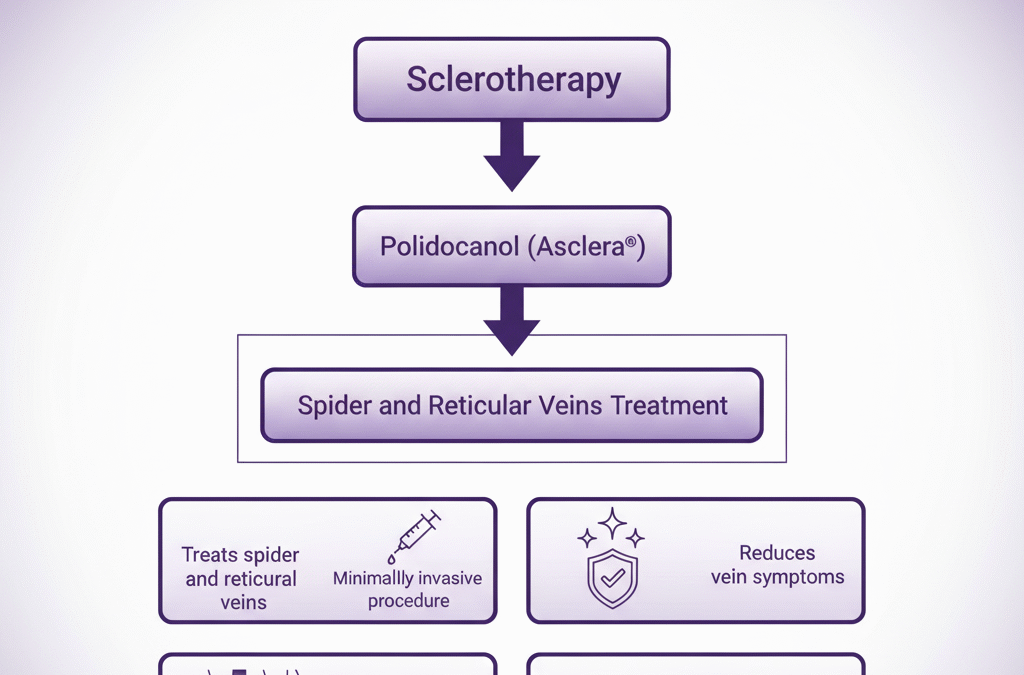
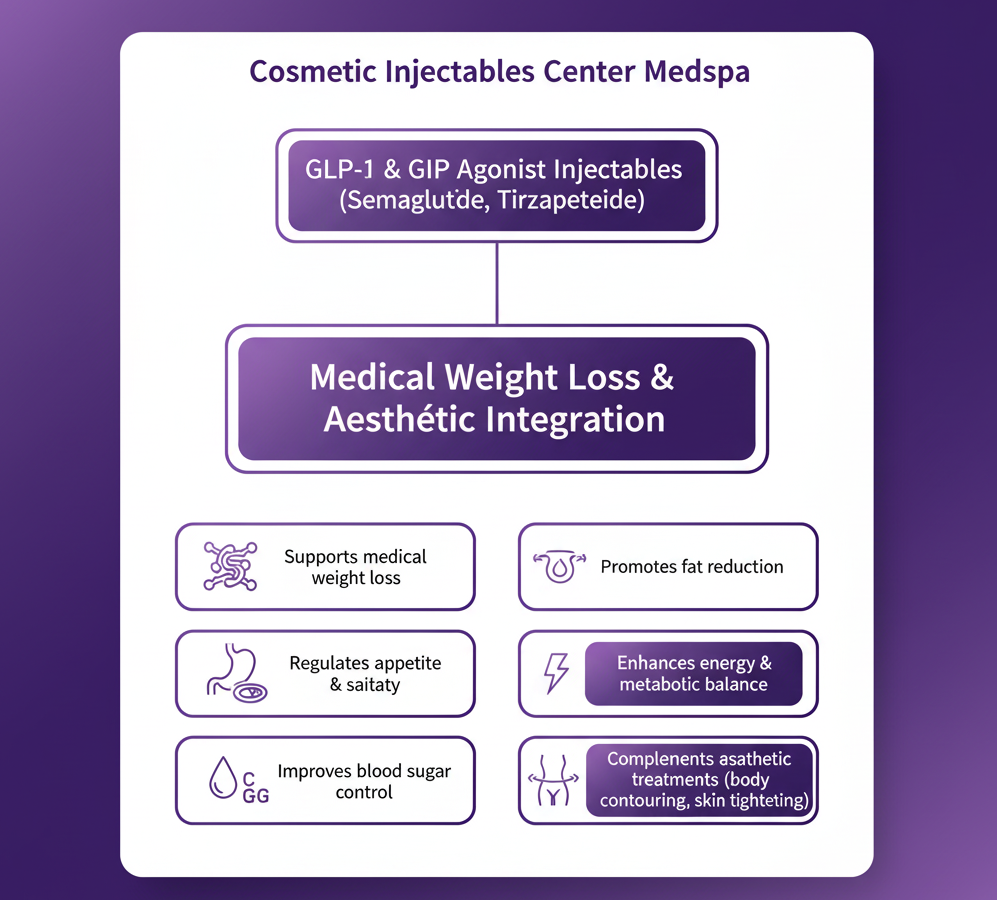
Jeuveau (prabotulinumtoxinA-xvfs) and Botox (onabotulinumtoxinA) are both FDA-approved neuromodulators for the temporary improvement of moderate to severe glabellar lines. While their mechanisms are similar, recent clinical research highlights distinct differences in...